Streamlining Clinical Trials: Miracle's Revolutionary Approach to Accelerating Medical Innovation
In the fast-paced world of biotech and pharmaceuticals, time is of the essence when it comes to bringing life-saving treatments to patients in need. However, the journey from drug development to market approval is often marred by complex challenges, lengthy delays, and soaring costs. Recognizing the urgency for change, Miracle, a dynamic startup founded in 2022 and headquartered in San Francisco, has emerged as a transformative force in the realm of clinical trials. By seamlessly connecting clinical trial data sources into a comprehensive dashboard, Miracle empowers biotech and pharma companies to accelerate their trials, revolutionizing the landscape of medical innovation. In this article, we will explore the pressing obstacles faced by the industry, delve into Miracle's groundbreaking solutions, and unveil the remarkable impact that streamlined connectivity can have on the future of clinical trials. Join us as we embark on a journey of discovery, where cutting-edge technology meets the pursuit of expedited patient care.
Introducing Miracle - Revolutionizing Clinical Trials with Streamlined Data Connectivity
Clinical trials play a pivotal role in the biotech and pharmaceutical industry, serving as the gateway for bringing new drugs to market. However, these trials frequently encounter formidable challenges, resulting in delays and escalating costs. Recognizing the need for a transformative solution, Miracle, a cutting-edge startup established in 2022 and headquartered in the heart of San Francisco, has set out to revolutionize the clinical trial landscape. By seamlessly connecting disparate clinical trial data sources into a single, comprehensive dashboard, Miracle empowers biotech and pharma companies to accelerate their trials, saving invaluable time, optimizing resources, and providing actionable insights that propel medical innovation forward.
In the realm of drug development, clinical trials represent a critical phase, where extensive testing and evaluation are conducted to ensure the safety, efficacy, and regulatory compliance of potential treatments. However, the complexity and fragmented nature of clinical trial operations often present significant hurdles, impeding progress and hindering the timely delivery of life-saving therapies to patients in need. These challenges range from the lack of cohesive communication between various software tools utilized by biotech companies to the inadequate transparency and inefficiencies in patient recruitment processes.
Miracle recognizes the pressing need to overcome these obstacles and streamline clinical trial operations by bridging the gaps that exist within the existing ecosystem. By seamlessly integrating diverse clinical trial data sources, Miracle constructs a unified platform that empowers biotech and pharma companies to navigate their trials with unprecedented efficiency. The amalgamation of this comprehensive dashboard significantly reduces administrative burdens, eliminates time-consuming manual data compilation, and liberates clinical operations teams to concentrate on their primary objective – advancing medical research and enhancing patient outcomes.
The transformative power of Miracle lies in its ability to provide actionable insights, arming sponsors with the necessary tools to make informed decisions that drive the acceleration of clinical trials. By consolidating vast quantities of trial-related data into a coherent, accessible format, Miracle's platform grants stakeholders unparalleled visibility into the entire trial process. Gone are the days of painstakingly sifting through multiple vendor portals and grappling with disjointed visualizations. Miracle streamlines this workflow, empowering sponsors to analyze real-time progress, assess recruitment performance, and optimize their strategies for maximum efficiency.
Moreover, Miracle understands the profound impact that time holds within the realm of clinical trials. With each passing day, potential revenue opportunities dwindle, and patients yearning for innovative treatments are left waiting. By implementing Miracle's data connectivity solutions, biotech and pharma companies can unlock the hidden potential of time, efficiently navigating the intricate web of clinical trial operations and swiftly bringing life-changing therapies to market. A single day saved through streamlined processes and enhanced recruitment strategies can translate into millions of dollars in additional patent-covered revenue, creating a significant competitive advantage within the industry.
As biotech and pharma companies strive to meet the demands of a rapidly evolving healthcare landscape, Miracle stands as a beacon of innovation and efficiency. With its commitment to revolutionizing clinical trials through streamlined data connectivity, Miracle is poised to shape the future of drug development, bringing hope and healing to countless individuals worldwide.
In the upcoming sections, we will delve deeper into the challenges faced by the biotech and pharmaceutical industry, the innovative solutions offered by Miracle, and the remarkable benefits that await those who embrace this groundbreaking approach. Join us as we explore the transformative power of Miracle in expediting clinical trials, revolutionizing patient care, and propelling medical advancements to new frontiers.
Meet the Founder - Jin Kim
At the helm of Miracle is Jin Kim, the Founder and CEO. With a background as a former athlete on the USA Archery Team and an education from MIT, Jin brings a unique perspective to the healthcare industry. Drawing on his experience in precision and focus from archery, he is committed to improving healthcare by bringing science to life and into the hands of patients more quickly.
Unraveling the Roadblocks: The Delays and Inefficiencies Plaguing Clinical Trials
Biotech and pharmaceutical companies face significant hurdles when conducting clinical trials. These trials are necessary to obtain FDA approval for marketable drugs, but they come with substantial costs and time investments. On average, it takes over $2 billion and 7-10 years to bring a new drug to market, leaving a limited window of exclusivity under patent protection.
One of the primary causes of delays in clinical trials is patient recruitment. More than 80% of trials experience recruitment challenges, hindering progress and jeopardizing the potential return on investment (ROI). Vendors and research sites often lack transparency, making it difficult for sponsors to account for ROI and optimize their trial's efficiency. Clinical operations teams spend a significant amount of time manually compiling data from various sources, creating visualizations, and reporting progress to management and the board.
Seamless Transformation: Consolidating and Automating Clinical Trial Reporting for Accelerated Progress
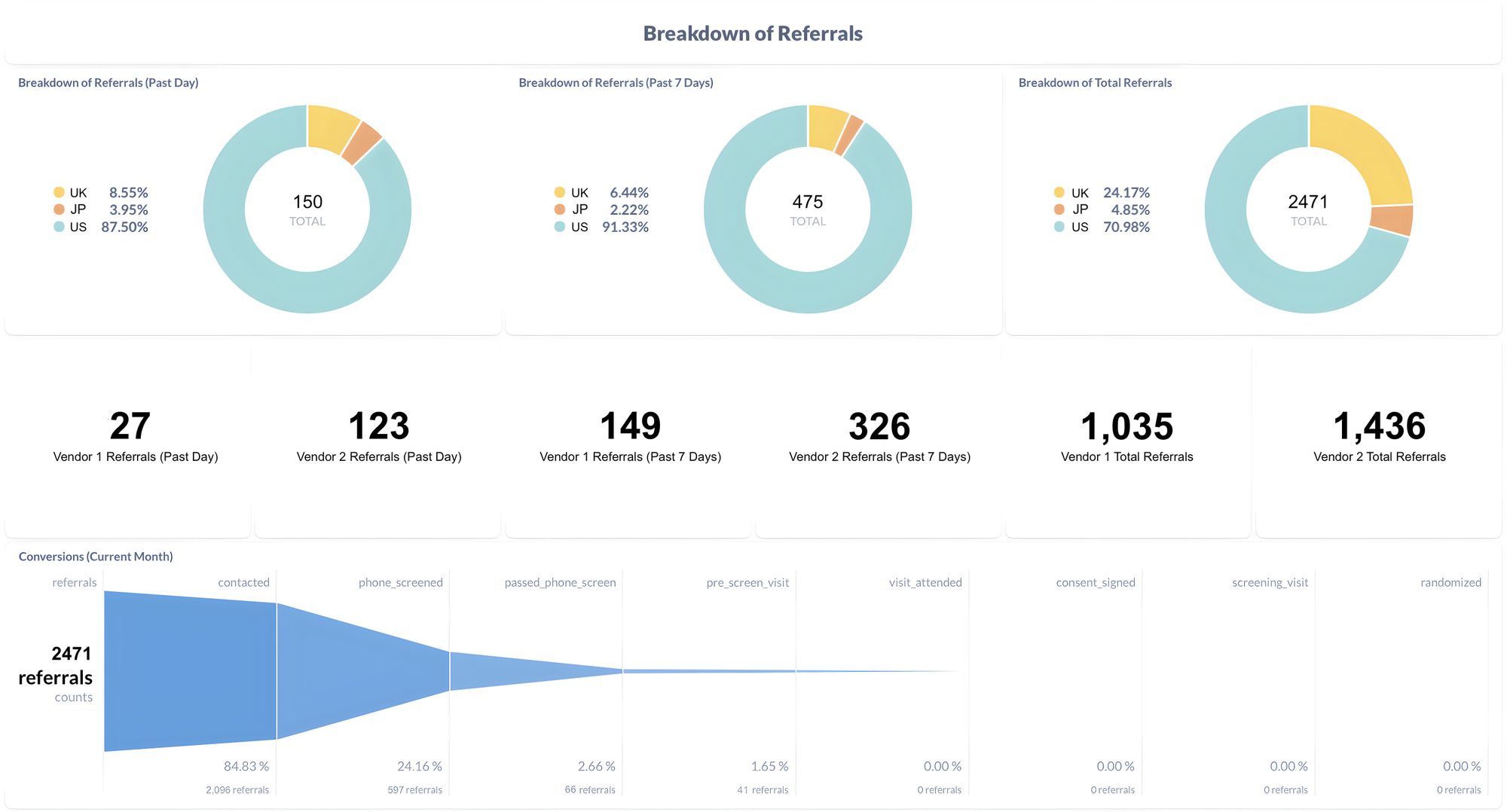
Miracle provides a comprehensive solution to streamline clinical trial reporting for biotech and pharma companies. By integrating with vendors and research sites, Miracle eliminates the need for manual data aggregation and report creation. This automation frees up valuable time for clinical operations teams, enabling them to focus on patient recruitment and improving study operations.
The key advantage of Miracle is its ability to consolidate and provide transparent reporting. With a single dashboard, sponsors can gain real-time insights into the progress of their trials. This transparency allows them to optimize their spending on vendors and research sites, ensuring they allocate resources effectively to expedite the trial process.
Unlocking the Value of Time - The Benefits of Miracle
Miracle offers significant benefits to biotech and pharma companies aiming to accelerate their clinical trials. By connecting patient recruitment data sources, Miracle enables sponsors to identify opportunities to optimize their trial operations. Even shaving off a single day from a trial can result in an average additional revenue opportunity of $2 million, making time a valuable asset in the pharmaceutical industry.
With Miracle's software, sponsors can access actionable insights that drive decision-making and expedite trial progress. By eliminating the manual compilation of information, clinical operations teams can focus on patient recruitment, ultimately increasing the efficiency of their studies. Moreover, the consolidated reporting provided by Miracle empowers sponsors to make data-driven decisions, allocating resources more effectively and reducing unnecessary spending.
The Future of Clinical Trials with Miracle
Miracle's mission is to revolutionize the clinical trial process, making it faster, more efficient, and ultimately improving patient access to innovative treatments. By harnessing the power of data integration and automation, Miracle aims to become the go-to platform for biotech and pharma companies seeking to accelerate their clinical trials.
As the startup continues to grow, Miracle plans to expand its reach, forge partnerships with key industry players, and enhance its software capabilities. By staying at the forefront of technological advancements and understanding the evolving needs of the industry, Miracle is well-positioned to drive innovation in clinical trials and shape the future of healthcare.
Conclusion
Miracle's innovative approach to streamlining clinical trial reporting holds tremendous potential for biotech and pharma companies. By connecting data sources into a single dashboard, Miracle accelerates trial progress, reduces costs, and empowers sponsors to make informed decisions.
As the demand for faster drug development and improved patient access increases, solutions like Miracle are vital in transforming the clinical trial landscape. By optimizing the use of resources and providing actionable insights, Miracle contributes to a more efficient and patient-centric healthcare system.
With its strong foundation, visionary leadership, and dedication to revolutionizing clinical trials, Miracle is poised to make a lasting impact on the biotech and pharmaceutical industry.